Our Knee Products
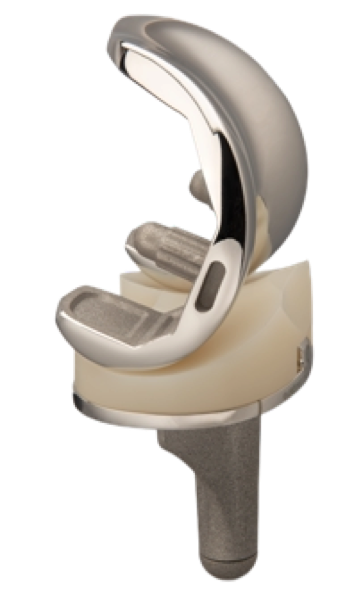
Quadrofit Total Knee System
The Quadrofit Total Knee System is designed and developed for use in cemented and cementless application of Total Knee arthroplasty. The unique femoral cutting guide combines various functionalities in one component. This enables the surgeon to shape the femur fast and accurate with only one guide positioning and fixing.
Quadrofit Total Knee System offers to a user/surgeon a lot of options used for application of total knee arthroplasty:
- Standard and Posterior Stabilized for Femur
- Fixed and Mobile Bearing for Tibia
- Fixed and Posterior Stabilized Inserts
- Extension stem and Augmentation for Tibia
- ReSurfacing and Insert Patella
All options can be performed with a single instrument set and offer intraoperative flexibility during surgery combined with maximum versatility.
Material:
Total Knee System manufactured from:
CoCrMo alloy according to ASTM F 75 – ISO 5832/4 for Femoral Component and Mobile Tibial Component Ti6Al4V ELI alloy according to ASTM F 136 – ISO 5832/3 for Fixing Tibial Component XL UHMWPE (Cross-Linked Ultra High Molecular Weight Polyethylene) according to ISO 5834/1-2 for Tibial Insert and Patella.
Quadrofit Revision Knee System
Quadrofit Revision Knee System is indicated for cases of revision of a primary total knee replacement or for primary total knee replacement when bone augmentation is required. Primary total knee arthroplasty may be subject to failure for various reasons, including polyethylene wear, aseptic loosening, osteolysis, infection, ligamentous instability and patellofemoral complications. Goals of a successful total knee revision include, amongst others: - Mechanical alignment restoration- Re-establishment of the joint line- good fixation of revision implant components- Acceptable range of motion restoration- Flexion/extension gap balancing It is the surgeon's responsibility to assess whether the indications for use are respected.
Material:
CoCrMo alloy according to ASTM F75-ISO 5832/4 for Revision Femoral Component.Classification:MDD 93/42/EEC, Annex IX, Rule 8, Class III
Indication of Use
The Quadrofit Revision Knee prosthesis is designed for cemented use in total knee arthroplasty, if there is evidence of sufficient sound bone to seat the support the components.
This knee replacement system is indicated in the following cases:
- Avascular necrosis of femoral condyle
- Post traumatic loss of joint configuration
- Primary implantation failure


Our Products

The name Euromed stands for high-quality joint implants and surgical instruments. Learn more about our Products.
Contact us
Euromed Implants GmbH
Headquarter
Bei St. Wilhadi 1
21682 Stade
Germany
Warehouse / Logistics
Große Schmiedestraße 18
21682 Stade
Germany
Fax +49 (0) 4141 7782 700
© 2026 - Euromed Implants GmbH
b. St. Wilhadi 1, 21682 Stade, Germany office@euromed-implants.de Tel. +49 (0) 4141 7897 290